NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Bookshelf ID: NBK369 PMID: 21250210
Definition
Skin testing for tuberculosis (TB) utilizes a form of the diagnostic reagent tuberculin. The multiple puncture technique is referred to as the Heaf, or tine test, whereas purified protein derivative (PPD-S) is commonly administered by intradermal injection, called the Mantoux technique after Charles Mantoux, who described the technique in the early part of the twentieth century. Old Tuberculin is used clinically only in the multiple puncture technique. PPD-S can also be used in some systems for multiple puncture, but the standard PPD-S solution should never be used in the multiple puncture technique as its concentration is not standardized for the technique. In the multiple puncture systems, only PPD-S designed for that system should be used. PPD-S occasionally is administered as "first strength" (1 TU) or "second strength" (250 TU) and is equivalent to a 1:100 concentration of OT. The clinical utility of first- and second-strength tuberculin is poorly defined. Intermediate-strength PPD-S remains the best studied. PPD-S, even when stabilized with Tween-80, is sensitive to light and to a certain extent to temperature. Thus it should be stored in a refrigerator.Technique
The multiple puncture technique uses either an applicator coated with dried tuberculin reagent or a similar device coated with liquid tuberculin immediately before application. The Heaf technique uses an injector. The applicator is pressed into the skin. The reaction is read at 48 and 72 hours. If discrete papules are present, the diameter of the largest is recorded. If vesiculation occurs, this is recorded separately and considered a positive test. If papules coalesce, the diameter of the largest area of induration is recorded. Although the multiple puncture technique has found favor as a screening tool, a positive result should be confirmed by Mantoux testing unless vesiculation has taken place. Thus, Mantoux testing is the procedure of choice. Mantoux testing has the advantage of using a standard amount of a standard potency reagent and thus is quantifiable and reproducible.The utility of the Mantoux technique is largely a function of the skill of the person administering the test and thus it should not be delegated to the least-experienced or least-trained member of the medical care team. The goal of the procedure is to deliver precisely 0.1 ml of the PPD-S intradermally. Subcutaneous administration will result in rapid "washout" from the area without time for the development of a reaction. Too superficial an injection results in leakage of the reagent onto the skin and the delivery of less than 5 TU intradermally.
The injection is performed using a tuberculin syringe made either of glass or plastic and a less-than-half-inch needle, either 26 or 27 gauge. The location for the injection may be anywhere, but traditionally the volar or dorsal surface of the forearm, sufficiently far from superficial veins, is chosen. Because skin thickness varies, the same spot should be chosen for each repeat test. The skin is prepared with an alcohol swab. With the needle bevel upward, the skin is punctured shallowly and a 6 to 10 mm tense wheal is raised upon injection of 0.1 ml. If the injection is unsuccessful, it may be repeated immediately, usually on the other arm (Figure 47.1).
Traditionally, the test is read at 48 and 72 hours, although positive reactions persist for several days following the 3-day period. Large reactions may endure for a week. A positive reaction usually includes both induration of the skin and surrounding erythema. The erythema has no diagnostic significance and should be ignored. Induration should be measured precisely in millimeters in the transverse dimension. There are several methods for determining the precise degree of induration. The lesion may be "stroked" with the fingertip, marking the point at which the skin is raised. If the area of induration appears to merge with the surrounding normal skin, examination of the lesion with a light shined obliquely across the skin can delineate the degree of induration. A common mistake is to record the results simply as "negative" or "positive." This does not allow comparison of the reaction with subsequent measurements.
Basic Science
The description of tuberculin by Robert Koch in the waning years of the nineteenth century is one of the classics in infectious diseases. Although originally thought of as treatment for tuberculosis, the substance has found use only as a diagnostic reagent. Despite nearly 100 years of clinical use, however, tuberculin and the tuberculin reaction remain shrouded in mystery.Tuberculin is a mixture of antigens derived from Mycobacterium tuberculosis and is available in two forms. Old Tuberculin (OT) is prepared by heating a broth culture of M. tuberculosis and then filtering it. Seibert and Glenn in 1941 described the batch preparation of a purified protein derivative of OT that has been called PPD-S. The potency of PPD-S is measured in tuberculin units (TU) that reflect the reactivity in weight per unit volume as compared to Seibert's lot 49608, the International Standard on deposit with the Bureau of Biologics of the U.S. National Institutes of Health and with the Statens Serum Institute in Copenhagen, Denmark. The activity of PPD-S is measured in a phosphate-buffered solution, although commercially the substance is stabilized with the detergent Tween-80 to prevent adsorption of the active principle to glass containers and syringes. The standard PPD-S dose is 5 TU per 0.1 ml.
The PPD reaction exploits the delayed-type hypersensitivity reaction of a patient previously infected with mycobacteria. A positive reaction is correlated histologically by the presence of mononuclear cells at the site of injection. This influx begins within 24 hours and is usually complete by 72 hours. Although the pathogenesis of this reaction is not completely understood, it appears to require the release of lymphokines in the skin and the subsequent attraction of uncommitted mononuclear cells to the area. Thus the predominant cells present in the tuberculin reaction are not those previously sensitized to tuberculosis. The characteristic erythema and edema of the tuberculin reaction are thought secondary to the increase in vascular permeability consequent to the inflammatory reaction. Although erythema may occur early, within the first 24 hours, this should not be confused with the tuberculin reaction as the erythema generally subsides and is thought secondary to an immediate-type hypersensitivity response to contaminants in the reagent. Regardless of the method, and even the contention that the tuberculin reaction is an epiphenomenon, the correlation between tuberculin reaction and tuberculosis is not disputed.
The validity of the tuberculin reaction in diagnosing tuberculosis hinges on the meaning of a positive and a negative test. Even the definition of these two seemingly simple concepts produces vigorous debate. In its official statement on the tuberculin skin test, the American Thoracic Society offers definitions that have become standards, although the debate continues.
The definition of reactions to tuberculin rests on epidemiologic data. A reaction to the Mantoux test may be divided into two groups: false positive and true positive. For the purposes of this discussion, a true positive is one that accurately predicts infection or previous exposure to Mycobacterium tuberculosis. Thus, a false positive may well be a delayed-type hypersensitivity reaction to PPD-S with its concomitant histologic and immunologic features, but is not associated with infection by M. tuberculosis. Although data are sparse and the investigation difficult to perform, the consensus is that the most common false positive tuberculin reaction is caused by infection with mycobacteriae other than tuberculosis (MOTT). Although infections with MOTT are widespread, their contribution to human disease is small in comparison to M. tuberculosis. Mycobacterium kansasii can cause a pulmonary disease indistinguishable from that caused by M. tuberculosis. The Mycobacterium avium complex can cause a chronic pulmonary infection, and disseminated disease is seen in the acquired immune deficiency syndrome (AIDS). As a rule, MOTT causing pulmonary disease are clustered geographically. Also, generally speaking, a tuberculin reaction caused by infection with MOTT tends to be smaller than those elicited by infection with M. tuberculosis. Thus, the criteria for positivity of the tuberculin reaction must take geography into account.
It would seem that the ideal population in which to determine the diameter of a positive test is one with bacteriologic evidence of tuberculosis. However, studies performed in tuberculosis sanatoriums and reported by Nash and Douglas (1980) showed that 25% of patients with active pulmonary tuberculosis failed to respond to 5 TU of PPD with greater than 10 mm of induration. These patients were, for the most part, not anergic to other skin test antigens and thus a specific inhibitor to PPD reactivity was posited to exist. Skin test reactivity is known to return in patients concomitant with treatment, but as an acute phase diagnostic test, a 25% false negative test for PPD is significant.
A false negative test results when the procedure fails to yield a positive result in the presence of a phenomenon it is designed to detect. Besides the inhibitor described previously, causes of a false negative intermediate-strength PPD include concurrent viral infections such as rubella and rubeola, as well as some bacterial infections including brucellosis and typhoid fever. Live virus vaccines, such as measles, have been shown to inhibit the development of a positive test. Reticuloendothelial malignancy and steroid therapy likewise have been thought to decrease tuberculin reactivity. Sarcoidosis appears in older lists as causing tuberculin negativity. Return of tuberculin positivity has even been thought to signal quiescence of sarcoid. Sarcoid patients, in general, will have tuberculin positivity when they have tuberculosis, although the correlation is not as well established as in patients without sarcoid. Psychological factors have also been shown to influence tuberculin reactivity. A correctable cause of tuberculin false negativity is the improper storage or administration of the reagent, or improper interpretation of the test. Because of the failure of positive controls to yield a high concordance with the test, epidemiologic methods need to be considered to establish the "cut point" for a positive test. Ideally, these data would yield a clear bimodal distribution, yet this is not the case. The previously alluded to geographic variation in skin test reactivity blurs the region between a positive and a negative test in many areas. In an effort to analyze the relative contributions of MOTT to tuberculin reactivity in populations, surveys were conducted in navy recruits using multiple antigens including PPD-S, PPD-B (prepared from the Battey bacillus, M. intracellulare), PPD-G (from the "Gause" strain of M. scrofulaceum) and histoplasmin. Reactors to PPD-B tended to have 6 to 11 mm reactions to PPD-S. Most important, it was found that the risk of developing tuberculosis increased with the size of the PPD-S reaction. Numerous subsequent studies have shown that the MOTT skin test antigens, while useful in epidemiological studies, cannot be applied to diagnosis in individual patients.
Thus it appears that the PPD must be interpreted geographically. Of course the clinical history is important. Patients who have radiographic evidence of pulmonary tuberculosis or have a previous history of close contact with a tuberculosis case are more likely to have a bimodal distribution; in these patients, tuberculin negativity can be assumed with a test diameter of less than 5 mm. This will give a very small false negative rate. In the general population without evidence of tuberculosis or exposure, a cut point of 10 mm seems acceptable. In areas with endemic MOTT, a higher cut point, perhaps 15 mm, could be considered.
In summary, lowering of the cut point will reduce the number of people with tuberculosis who will be missed by testing (i.e., false negatives). This is at the expense, however, of including people with so-called positive tests who do not have the disease (i.e., false positives). The converse is true with raising the cut point: the false negative rate will be increased.
Since one of the values of the Mantoux test is the identification of new infections, the so-called converters, it is valuable to know that PPD-S is not, in itself, sensitizing. Delayed-type hypersensitivity to tuberculin, once established by whatever means, may decrease with advancing age as well as temporal distance from exposure to tuberculosis. These people may have an insignificant tuberculin reaction when initially tested, yet convert on retesting. This is termed the booster effect. The presence of boosted reactors in the population may yield an inordinately high conversion rate if not recognized. Because the booster phenomenon can be observed in as short a time as 1 week, Bass and Serio (1981) and others have suggested that repeat skin testing a short while after the detection of an insignificant reaction will identify those who "boost" into the positive range and eliminate them for consideration as converters in subsequent testing periods. If the repeat test falls into the positive range, the person can be managed in the manner routine for a positive reaction in the individual clinical situation. Previous vaccination with BCG may also cause a "false positive" reading. This, however, is variable, and the reaction also wanes with time. A BCG recipient with a positive tuberculin reaction should therefore be evaluated as if he or she had not received the vaccine.
Clinical Significance
Despite the variability and multiple caveats attendant to Mantoux testing, the PPD carries with it enormous clinical utility. The American Thoracic Society (1981) suggests the following list of persons in whom tuberculin testing is indicated:- Persons with signs (e.g., radiographic abnormality) and/or symptoms (cough, hemoptysis, weight loss, etc.) suggestive of current tuberculosis disease.
- Recent contacts with known tuberculosis cases or persons suspected of having tuberculosis.
- Persons with abnormal chest roentgenograms compatible with past tuberculosis.
- Persons with medical conditions that increase the risk of tuberculosis (silicosis, gastrectomy, diabetes, immunosuppressive therapy, lymphomas, etc.).
- Groups at high risk of recent infection with M. tuberculosis, such as immigrants from Asia, Africa, Latin America, and Oceania; some inner-city and "skid-row" populations; personnel and long-term residents in some hospitals, nursing homes, mental institutions and prisons.
At present, testing with second-strength tuberculin does not appear useful because it is more likely to yield a false positive from cross-reactivity than to uncover a covert reactor. Two-stage testing with intermediate-strength PPD-S to exploit the booster phenomenon is probably more useful in that situation.
In an epidemiological study of the efficacy of tuberculosis control, population tuberculin screening may be useful. However, as a general situation in an area of low endemicity such as the United States, population screening is likely to be complicated by cross-reactivity and the booster phenomenon.
Use of the PPD Test in Acquired Immune Deficiency Syndrome (AIDS)
Both M. tuberculosis and the M. avium complex are seen in patients with AIDS. Other MOTT, such as M. kansasii, have been seen in this setting as well. The Centers for Disease Control 1987 revision of the AIDS case definition cites disseminated infection with M. avium complex or M. kansasii as AIDS defining conditions. In the presence of laboratory evidence for infection with the human immune deficiency virus (HIV), any disseminated MOTT or extrapulmonary tuberculosis becomes AIDS defining. As the role of mycobacteria in AIDS becomes more widely recognized, diagnostic tests for these conditions assume greater importance.Although patients with AIDS commonly are anergic to PPD and may not form well-defined granulomata, patients who are simply HIV antibody positive may respond to PPD (Sunderam et al., 1986). The American Thoracic Society now suggests that a PPD test should be applied via the Mantoux technique using a standard 5 TU dose (Snider et al., 1987). In the presence of a significant positive test, the patient should be evaluated for the presence of M. tuberculosis. The American Thoracic Society suggests that a 12-month course of isoniazid should be considered for such patients. Patients with HIV-related illnesses other than tuberculosis should likewise receive a PPD test, keeping in mind that a false negative result may be observed. A negative test in no way excludes tuberculosis in this setting.
The relationship between a positive PPD and the development of tuberculosis has been underscored by Selwyn and colleagues (1989), who studied HIV-infected intravenous drug users. When compared with non-HIV-infected controls, the HIV-infected patients with positive PPDs (> 10 mm) had a substantially higher risk of developing tuberculosis during the study period. This investigation lends further urgency to the recommendation to perform PPD testing on all HIV-infected patients and persons at risk for acquiring the virus. The latter is important because the virus appears to cause cutaneous anergy in many patients.
References
- American Thoracic Society. The tuberculin skin test. Am Rev Respir Dis. 1981;124:356–63.
- Bass JB, Serio RA. The use of repeat skin tests to eliminate the booster phenomenon in serial tuberculin testing. Am Rev Respir Dis. 1981;123:394–96. [PubMed: 7224351]
- Centers for Disease Control. Revision of the CDC surveillance case definition for acquired immune deficiency syndrome. MMWR. 1987;36(suppl 1S):4S–5S.
- David HL, Selin MJ. Immune response to mycobacteria. In: Rose NR, Friedman H, eds. Manual of clinical immunology. Washington, DC: American Society for Microbiology, 1980;520–25.
- Editorial. New tuberculins. Lancet 1984;1:199–200.
- McMurray DN. Mechanisms of anergy in tuberculosis. Editorial. Chest. 1980;77:4–5. [PubMed: 7351144]
- Muller HK, Pye DW, Martin CL. et al. Tuberculin anergy in clinically normal individuals I. Lymphokine and lymphocyte transformation studies. Int Arch Allergy Appl Immunol. 1983;70:65–70. [PubMed: 6336724]
- Nash DR, Douglas JE. Anergy in active pulmonary tuberculosis, a comparison between positive and negative reactors and an evaluation of 5 TU and 250 TU skin test doses. Chest. 1980;77:32–37. [PubMed: 7351142]
- Reichman LB. Tuberculin skin testing, the state of the art. Chest. 1979;76(6suppl):764–70. [PubMed: 510024]
- Seibert FB, Glenn JT. Tuberculin purified protein derivative preparation and analysis of a large quantity for standard. Am Rev Tuberc. 1941;44:9–25.
- Selwyn PA, Hartel D, Lewis VA. et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. New Engl J Med. 1989;320:545–550. [PubMed: 2915665]
- Smith GR, McDaniel SM. Psychologically mediated effect on the delayed hypersensitivity reaction to tuberculin in humans. Psychosom Med. 1983;45:65–70. [PubMed: 6844530]
- Snider DE, Hopewell PC, Mills J. et al. Mycobacterioses and the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1987;136:492–96. [PubMed: 3304048]
- Sunderam G, McDonald RJ, Maniatis T. et al. Tuberculosis as a manifestation of the acquired immunodeficiency syndrome (AIDS). JAMA. 1986;256:362–66. [PubMed: 3723722]
Figures
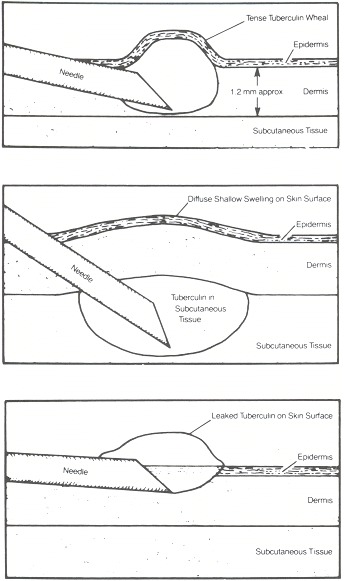
Figure 47.1
(A) A properly inserted needle. (B) The needle is too deep. (C) The needle is too shallow. (Reproduced with permission from Guidelines for the Diagnosis of Tuberculous Infection. Copyright © 1984, Parke-Davis, Division of Warner-Lambert Company.)
Copyright © 1990, Butterworth Publishers, a division of Reed Publishing.
Không có nhận xét nào:
Đăng nhận xét