NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Bookshelf ID: NBK445 PMID: 21250266
Definition
Tests for fecal occult blood detect blood in the stool that is not visible on gross inspection, usually less than 50 mg of hemoglobin per gram of stool. Normal adults usually show less than 2 to 3 mg/gm. Increased amounts are associated with a variety of benign and malignant gastrointestinal diseases, especially colonic neoplasms, and the tests are most often used in screening patients for such lesions.Technique
Benzidine-based tests (e.g., Hematest) have been virtually eliminated from use because of their excessive sensitivity, which results in a high frequency of false-positive reactions, as well as the carcinogenicity of the reagent.The current worldwide standard is the Hemoccult slide test (Smith-Kline Diagnostics), a guaiac-based test, in which one side of a guaiac-impregnated paper is smeared with stool and tested by addition of a few drops of developer solution (stabilized peroxide reagent) to the opposite side of the paper. In screening programs, the patient recovers stool from the toilet bowl using a wooden applicator, smears a small portion onto two windows of the card, and closes the cover. This is done on three successive days, and the cards are mailed for testing, as there is little degradation of reactive hemes in the dry, smeared specimens over a period of 1 week (fewer than 15% of samples).
Appearance of unequivocal blue color, of any intensity, within 30 seconds, is considered a positive test; greenish colors, caused by oxidation of fecal bilirubin to biliverdin, should not be read as positive. Positive and negative control spots are included in the card to be sure that the reagents are reactive with substrate and not reactive in the absence of substrate. It has been found that diffusion of the hematin from the smeared stool into the paper is important, so that the reaction can be enhanced by wetting the smeared side of the paper with a few drops of water several minutes before adding the developer. However, such rehydration of specimens also increases the rate of false-positive reactions from plant peroxidases and meat hemes in the diet, and is not recommended unless there is rigid adherence to a restricted diet, outlined in Table 98.1.
A modification of the Hemoccult test has been marketed (ColoScreen, Helena Laboratories) in which a selective inhibitor of nonheme vegetable peroxidases has been added in hopes of diminishing the false-positive reactions from this source. Another group of tests is based on the immunologic detection of human hemoglobin or globin in the stool, but the tests developed thus far have been unreliable because of over- or undersensitivity and poor reproducibility. Both approaches might improve specificity, but neither solves the problem of major and variable degradation of hematin or globin in the intestinal lumen, a problem shared by Hemoccult.
A new, promising, and almost quantitative approach (HemoQuant, Bioscience Labs) is to measure fecal hemes by the specific fluorescence of their porphyrin derivatives, after extraction of the porphyrins from the specimen to remove interfering substances. A variable (1 to 98%) proportion of unabsorbed hemoglobin/hematin is converted to porphyrins by intestinal bacteria (so-called intestinally converted fraction, or ICF); this fraction can be extracted into an organic solvent in the presence of citric acid. The remaining intact hemes can be chemically converted to porphyrins by heating in a test tube in the presence of oxalic acid and ferrous sulfate, and then extracted, along with the ICF, to yield total converted porphyrins as a measure of total unabsorbed hemes.
HemoQuant is little affected by the variable degradation of hemes in the intestinal lumen or by the degree of hydration of the stool; is insensitive to nonheme peroxidases, oral iron, and cimetidine; and is almost quantitative. It is therefore highly likely to become the future test of choice, if it can be simplified and its cost reduced. The early hope, that the ICF would help to distinguish upper from lower gastrointestinal blood loss, has not been realized.
Neither HemoQuant, any of the variants of the other new tests, nor the numerous commercial imitations of Hemoccult, have undergone the extensive evaluation in large screening programs that has documented the efficacy and identified the limitations in interpretation of the Hemoccult test. Moreover, the appropriate upper normal limits for fecal hemes have not been established for these new tests. For the present, therefore, Hemoccult is the only validated test appropriate for use in detection of fecal occult blood.
Basic Science
As shown in Figure 98.1, erythrocytes entering the gastrointestinal lumen are usually lysed to free hemoglobin, which is converted to free hematin (Fe3+ state) plus globin. Up to 15% of the hematin is absorbed and converted to bilirubin by microsomal heme oxygenase in the enterocytes. The remaining 85% may be further degraded, principally by colonic bacteria, to form porphyrins. The globin is digested by pancreatic and intestinal mucosal proteases to yield peptides and amino acids.Many tests have been designed to detect hemoglobin or its intestinal metabolites in the stool (Figure 98.1). Thus, blood released into the gastrointestinal tract can be detected as hemoglobin (51Cr-labeled red cells, immunoassay), hematin (peroxidase activity), porphyrins (fluorescence), or globin (immunoassay). Most such tests have inherent unreliability, however, because the proportion of hemoglobin degraded varies greatly among individuals. The only truly quantitative tests are: (1) radioassay of 51Cr in the stool after intravenous administration of autologous red cells labeled with 51Cr-chromate on the β-chain of hemoglobin (the chromate is neither degraded nor absorbed in the intestine); and (2) measurement of total fecal porphyrins after chemical conversion of all fecal hemoglobin to porphyrins (HemoQuant test). Unfortunately, these two tests are complex, cumbersome, time-consuming, and expensive. Consequently, the most widely used, established methods rely on the detection of undegraded hematin and hemoglobin by virtue of their peroxidase-like activity.
Peroxidase-based tests (Figure 98.2) involve the addition to a stool sample of a peroxide as substrate and guaiac, benzidine, or related compounds as a redox indicator. The peroxidase activity of hematin and/or hemoglobin catalyzes the oxidation of the colorless indicator compound to a blue quinone. False-positive reactions are given by other materials that can catalyze the peroxidase reaction (Table 98.1), including dietary peroxidases in vegetable and fruits, hemoglobin and myoglobin in red meats (especially if rare or raw); oral iron, once thought to be a problem, has been shown clearly not to affect the Hemoccult test. Cimetidine also gives false-positive reactions, but only in aspirates of gastric arid duodenal juice, because most of this drug is absorbed before reaching the colon. False-negative tests occur if high concentrations of reducing agents are present in the diet, especially ascorbic acid in citrus fruits and juices in doses of 750 mg or more daily. Drugs such as aspirin and nonsteroidal anti-inflammatory agents, which often cause erosions and bleeding of otherwise normal gastric mucosa, also lead to positive tests that may falsely suggest the presence of an intrinsic lesion. It is therefore important that intake of these interfering substances be eliminated for 3 days before and 3 days during collection of the stools to be tested.
Clinical Significance
Colonic neoplasms and other gastrointestinal lesions bleed intermittently and in variable amounts from day to day. Therefore, if any of the six windows (2 per stool × 3 stools) yields a blue reaction, the patient should undergo more intensive diagnostic study to determine the source of blood loss. In Western nations, Hemoccult screening of truly asymptomatic populations aged 45 years or more has yielded 3 to 5% positive tests on an unrestricted diet and less than 2% positive tests on the restricted diet (Table 98.1). On the latter diet, 10 to 15% of those with positive tests will be found to have carcinoma and 20 to 30% to have benign neoplasms (e.g., polyps), usually in the colon. Another 30 to 60% of positive subjects will reveal other gastrointestinal lesions that might be responsible for occult bleeding (e.g., hemorrhoids, diverticulosis, inflammatory bowel disease, peptic ulcer, gastritis, esophagitis, and esophageal varices). Less than 15%, and usually less than 10%, of positive subjects will reveal no lesions, and most of these probably have false-positive tests due to vegetable peroxidases from the diet.Sixty-five to 80% of colorectal carcinomas and 20 to 40% of benign colonic adenomas are detected by the three-stool Hemoccult test. Thus, any one positive stool is an indication for a full work-up of the entire colon, including either air-contrast barium enema plus flexible (60 cm) sigmoidoscopy, or full colonoscopy. Flexible sigmoidoscopy or rigid proctoscopy alone are satisfactory as complementary tests with Hemoccult in screening programs but are inadequate for further evaluation of a Hemoccult-positive stool because over one-third of colonic neoplasms are proximal to the rectosigmoid. If only benign lesions or no lesions are discovered after full examination of the colon, upper gastrointestinal endoscopy is probably indicated, though its cost effectiveness has not been validated. If still nothing is found, it is probably best to evaluate if the Hemoccult test is a false-positive, using 51Cr-labeled erythrocytes or the HemoQuant test to quantify fecal blood loss. If the presence of bleeding is confirmed, one may consider performance of a small bowel series, mesenteric angiography, and/or abdominal scintiscan after intravenous administration of 99mTc-labeled erythrocytes. Unfortunately, these tests have a low yield in the patient with occult blood loss. The fluorescein string test for upper gastrointestinal blood loss has a high frequency of false-positive results and should not be used.
Most of the colorectal neoplasms detected with Hemoccult screening will be either benign adenomas or Duke's stage A or B1 carcinomas, with an 80% or more 5-year survival after resection. By contrast, in patients with symptomatic carcinoma, the proportion of 5-year survivals is less than 40%. Because of built-in biases in screening programs, however, one may merely be making the diagnosis at an earlier stage in the disease, without actually postponing the time of demise. Therefore, it is not yet proven that screening for colon cancer actually improves survival; long-term, randomized, prospective studies, well under way in New York and Minneapolis, should answer this important question.
References
- Ahlquist DA, McGill DB, Schwartz S. et al. HemoQuant, a new quantitative assay for fecal hemoglobin. Comparison with Hemoccult. Ann Intern Med. 1984;101:297–302. [PubMed: 6465700]
- Ahlquist DA, McGill DB, Schwartz S. et al. Fecal blood levels in health and disease. A study using HemoQuant. N Engl J Med. 1985;312:1422–28. [PubMed: 3873009]
- Caligiore P, MacRae FA, St, John DJB. et al. Peroxidase levels in food: relevance to colorectal cancer screening. Am J Clin Nutrit. 1982;35:1487–1489. [PubMed: 7081130]
- Eddy DM, Nugent FW, Eddy JF. et al. Screening for colorectal cancer in a high-risk population. Results of a mathematical model. Gastroenterology. 1987;92:682–92. [PubMed: 3102307]
- Ellefson BS, Larson A, Schwartz S. et al. The HemoQuant test, remodelled for the busy clinical laboratory. Clin Chem. 1986;32:1142–43.
- Gilbertsen VA, McHugh RB, Schuman L. et al. The earlier detection of colorectal cancers. A preliminary report of the results of the occult blood study. Cancer. 1980;45:2899–2901. [PubMed: 7379021]
- McDonnell, WM, Ryan JA, Soeger, DM, Elta, GH Effect of iron on the guaiac reaction. Gastroenterology. 1989;96:74–78. [PubMed: 2909440]
- MacRae FA, St, John DJB. Relationships between patterns of bleeding and Hemoccult sensitivity in patients with colorectal cancers or adenomas. Gastroenterology. 1982;82:891–98. [PubMed: 7060910]
- MacRae FA, St John JB, Caligiore P. et al. Optimal dietary conditions for Hemoccult testing. Gastroenterology. 1982;82:899–903. [PubMed: 7060911]
- Morris DW, Hansell JR, Ostrow JD. et al. Validity of fecal occult blood tests in hospitalized patients. Am J Dig Dis. 1976;21:845–52. [PubMed: 1087829]
- Ostrow JD, Mulvaney CA, Hansell JR. et al. Sensitivity and reproducibility of chemical tests for fecal occult blood. Am J Dig Dis. 1973;18:930–40. [PubMed: 4749195]
- Peterson WL, Fordtran JS. Editorial. Quantitating the occult. N Engl J Med. 1985;312:1448–50. [PubMed: 3873010]
- Schwartz S, Dahl J, Ellefson, M et al. The HemoQuant test: a specific and quantitative determination of heme (hemoglobin) in feces and other materials. Clin Chem. 1983;29:2061–67. [PubMed: 2909440]
- Simon JB. Gastroenterology. 1985;88:820–37. [PubMed: 3917961]
- Winawer SJ, Andrews M, Flehinger B. et al. Progress report on controlled trial of fecal occult blood testing for the detection of colorectal neoplasia. Cancer. 1980;45:2959–64. [PubMed: 6992968]
Figures
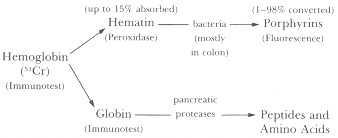
Figure 98.1
Degradation of hemoglobin in the gastrointestinal tract and methods to detect hemoglobin and its metabolites in feces.
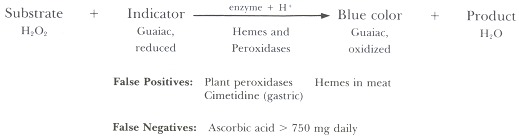
Figure 98.2
Principle of peroxidase-based tests.
Tables
Table 98.1Strict Low-peroxidase Diet for Occult Blood Screening
| Do not ingesta | ||
| Rare red meat | Carrot | Mushrooms |
| Bloodwurst | Cauliflower | Parsnip |
| Horseradish | Cucumber | Pumpkin |
| Broccoli | Grapefruit | Turnips |
| Cabbage | Green beans | Zucchini |
| Cantaloupe | ||
| Nonsteroidal anti-inflammatory agents | ||
| Ascorbic acid | ||
| Aspirin | ||
| O.K. to ingest | ||
| Well-cooked fish and chicken | Bran cereals | |
| Cooked fruits and vegetables | Peanuts | |
| Canned tuna fish | Popcorn | |
| Strawberries | Oranges (1 a day) | |
- a
- Less than 50 g of the foods listed have peroxidase activity equivalent to 1.0 ml blood (1 mg hemoglobin per gram of stool).
Copyright © 1990, Butterworth Publishers, a division of Reed Publishing.
Không có nhận xét nào:
Đăng nhận xét