NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Bookshelf ID: NBK352 PMID: 21250193
Definition
Creatine kinase (CK), formerly known as creatine phosphokinase, is an intracellular enzyme present in greatest amounts in skeletal muscle, myocardium, and brain; smaller amounts occur in other visceral tissues.Disruption of cell membranes due to hypoxia or other injury releases CK from the cellular cytosol into the systemic circulation. On this basis, elevated serum levels of CK have been used as a sensitive but nonspecific test for myocardial infarction. The poor specificity reflects the ubiquity of CK in many tissues other than the myocardium.
Because of the many current assay methods in use, there is no standard reference value for serum CK. Normal values are best determined locally based on the method employed and the ranges for healthy controls. Values are expressed in international units per liter.
CK is a dimeric molecule composed of two subunits designated M and B. Combinations of these subunits form the isoenzymes CK–MM, CK–MB, and CK–BB. A significant concentration of CK–MB isoenzyme is found almost exclusively in the myocardium, and the appearance of elevated CK–MB levels in serum is highly specific and sensitive for myocardial cell wall injury. Normal reference values for serum CK–MB range from 3 to 5% (percentage of total CK) or 5 to 25 IU/L.
Technique
CK catalyzes the reversible reaction: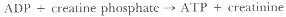
Separation of CK into isoenzymes may be accomplished by electrophoresis, column chromatography, or radioimmunoassay. Most clinical laboratories use electrophoresis on agarose gel or cellulose acetate combined with band quantification by fluorometric or spectrophotometric techniques. Quantification may also be accomplished by elution of the electrophoretic bands. Electrophoretically CK–BB is most mobile, CK–MB is intermediate, and CK–MM is neutral.
Although electrophoresis is possibly less sensitive than column chromatography or radioimmunoassay, there has been extensive experience and it is adequate for routine clinical use. The sensitive column chromatography method finds most use in research applications. Radioimmunoassay methods for isoenzymes can be accomplished rapidly and may be the method of the future with further development.
Basic Science
The advent of enzymology as a tool in the diagnosis of myocardial infarction may be credited to Karmen and associates (1955), who documented elevation of aspartate aminotransferase (AST, SGOT) and lactic dehydrogenase (LDH) in the serum of patients with acute myocardial infarction.Subsequently, there has been a search for enzyme methods that are readily performed in a clinical laboratory and permit an accurate, early, sensitive, and specific diagnosis of myocardial infarction. Does CK–MB detection and quantification fill this role?
As stated previously, determination of total CK levels suffers from lack of specificity. Trauma, surgery, vascular disease, and metabolic disease of the central nervous system may produce significant elevation of CK. Similarly, several diseases of muscle (e.g., trauma, metabolic disease such as myxedema, diabetic ketoacidosis, hypothermia, inflammatory disease such as polymyositis, and degenerative disease such as Duchenne's muscular dystrophy) may elevate CK levels. Of particular note is elevation of CK with intramuscular injections often given for chest pain.
When subjected to electrophoresis, the serum of healthy individuals contains predominately CK–MM isoenzyme. Sensitive methodology can detect traces of CK–MB and CK–BB.
The major locus of CK–MB is myocardium. Exact amounts are disputed, ranging from 15 to 30% CK–MB and 70 to 85% CK–MM. Older reports have indicated that CK–MB was absent from skeletal muscle, but radioimmunoassay enzyme analysis reports levels of CK–MB of 5 to 7% in skeletal muscle. This finding may account for abnormal levels of CK–MB reported in endurance and contact athletes. Other sources of significant CK–MB levels are rare.
Any process that disrupts cardiac sarcolemmal membranes (e.g., myocarditis, cardiac trauma, or cardiac surgery including endomyocardial biopsy) can release cytosolic CK–MB. Elevated serum levels of CK–MB are therefore specific for myocardial cellular injury, but not for acute myocardial infarction.
Following onset of symptoms of myocardial infarction CK and CK–MB increase in serum within 3 to 6 hours; the peak levels occur between 16 and 30 hours. Significantly, CK–MB disappears from the serum at a more rapid rate than CK. For example, CK–MB returns to normal by 24 to 36 hours, whereas the elevated CK levels may be detected for up to 60 hours. This "window" dictates that CK and CK–MB must be determined as soon as possible after the onset of symptoms, and repeated several times in the first 48 hours. Peak CK–MB levels range from 15 to 30% of total CK postinfarction.
Infarct size may be estimated by constructing a time-activity curve of appearance, peak, and disappearance of CK and/or CK–MB activity by use of the mathematical model of Roberts, Henry, and Sobel (1985). The validity of this method has been confirmed by demonstrating congruence of anatomical infarct size and the estimated value. This methodology has been of use in interventional studies of infarct-size reduction, but has not been widely employed clinically. Estimated infarct size is not an independent predictor of mortality following acute myocardial infarction.
CK enters the vascular space via cardiac venous and lymphatic drainage following disruption of cell membranes. Direct entrance into the ventricular cavity may occur because earlier CK rises are reported in non-Q wave infarction versus transmural infarction. Removal from the blood pool is believed to be by the reticuloendothelial system, which may explain the ability of some drugs to increase CK levels by prolonging CK half-life.
Following thrombolytic therapy, peak release of CK and CK–MB occurs several hours earlier and in larger amounts for given infarct size as opposed to patients treated conventionally. This phenomenon is thought to result from enzyme "washout" by reperfusion. It has been used as a noninvasive marker of reperfusion following intravenous thrombolysis.
Using prolonged electrophoresis and special focusing techniques, three subbands of CK–MM have been demonstrated in man and animal models following acute MI. These are termed CK–MM3, CK–MM2, and CK–MM1. Significant amounts of CK–MM3, are present only in myocardium, and when released into plasma are converted to CK– MM1, with an intermediate step through CK–MM2. Following myocardial infarction, CK–MM3, rises rapidly in plasma and exceeds CK–MM1, normally the dominant plasma subform. In experimental MI, CK–MM3, rose as early as 1 hour post-MI, peaked at 4 hours, and disappeared by 12 hours. Since CK–MM is present in skeletal muscle, this test may suffer from the same lack of specificity as CK levels. In the appropriate clinical setting, however, the test may allow very early diagnosis of myocardial infarction or infarct extension. Further study is necessary to demonstrate clinical utility.
Clinical Significance
The early and accurate diagnosis of acute myocardial infarction is obviously a desirable goal for assessment of symptoms and planning of therapy. Additionally, expensive coronary care unit time should be utilized in a cost-effective manner. Chest pain syndromes are not specific, and absolute ECG diagnosis ("Q wave infarction") is specific but insensitive.Determination of CK–MB isoenzyme has a 98% predictive value for myocardial necrosis with a positive enzyme profile and a 100% negative predictive value for the absence of necrosis with a normal profile. Values must be assessed within 24 hours of symptom onset, however.
For the optimal clinical use of CK–MB determinations, the following suggestions are made:
- The clinician must have access to a quality-controlled laboratory capable of determining CK and CK–MB isoenzymes rapidly and with an acceptable degree of reproducibility.
- Because of the short serum half-life of CK–MB, blood sampling must begin within 48 hours (and preferably within 24 hours) of symptoms. Serum sampling should be obtained at first contact and at 8- to 12-hour intervals for 48 hours to observe the characteristic appearance, peak, and disappearance of CK– MB. Serial sampling is emphasized and single values in the emergency room setting are inadequate to exclude myocardial injury.
- Patients seen more than 48 hours after symptoms should have determination of lactic dehydrogenase (LDH, total and isoenzymes), seeking the characteristic elevation of total LDH and isoenzyme "flip" seen after infarction (LDH-1 isoenzyme to LDH-2 isoenzyme in a ratio of 1.0 or more in the presence of increased total LDH levels). Peak LDH values occur 48 to 72 hours following infarction and remain abnormal for 10 to 14 days. False positives are not uncommon in the measurement of LDH and its isoenzymes. Major pitfalls include hemolysis of the blood sample, hepatic congestion due to CHF, and skeletal muscle damage.
- Clinical judgment must be exercised in the rare instance where CK–MB is elevated in the absence of myocardial necrosis. A prime example is the occurrence of chest pain in an athlete during or after competition, where CK–MB levels may be elevated (both percentages and absolute values) from skeletal muscle sources.
- When the diagnosis of acute MI is strongly entertained, CK–MB levels should be determined in spite of normal CK levels. Small infarctions may release significant levels of CK–MB with normal total CK. This situation is unusual, however, and further samples should be obtained and analyzed.
- Because of the rapid rise and fall of CK–MB levels after a myocardial infarction, it is useful to sample CK–MB again at 8- to 12-hour intervals following recurrent post-MI chest pain in order to detect infarct extension.
- Regardless of the sensitivity and specificity of CK–MB in detecting myocardial injury, the study has no diagnostic power for the diagnosis of severe ischemia without infarction. Clinical acumen and appropriate studies are necessary for accurate diagnosis.
References
- Blanke H, von Hardenberg D, Cohen M. et al. Patterns of creatine kinase during acute myocardial infarction after nonsurgical reperfusion: comparison with conventional treatment and correlation with infarct size. J Am Coll Cardiol. 1984;3:675–80. [PubMed: 6693639]
- Foreback CC, Chu JW. Creatine kinase isoenzymes: electrophoretic and quantitative measurements. CRC Crit Rev Clin Lab Sci. 1981;15:187–230. [PubMed: 7032842]
- Grande P, Christiansen C, Pedersen A. et al. Optimal diagnosis in acute myocardial infarction. A cost-effectiveness study. Circulation. 1980;61:723–28. [PubMed: 6766824]
- Grande P, Nielsen A, Wagner GS. et al. Quantitative influence of serum creatine kinase isoenzyme MB estimated infarct size and other prognostic variables on one year mortality after acute myocardial infarction. Br Heart J. 1985;53:9–15. [PubMed: 3966955]
- Hackel DB, Reimer KA, Ideker RE. et al. Comparison of enzymatic and anatomic estimates of myocardial infarct size in man. Circulation. 1984;70:824–35. [PubMed: 6488496]
- Hashimoto H, Abendschein DR, Strauss AW. et al. Early detection of myocardial infarction in conscious dogs by analysis of plasma MM creatine kinase isoforms. Circulation. 1985;71:363–69. [PubMed: 3965176]
- Jaffe AS, Garfinkel BT, Ritter CS. et al. Plasma MB creatine kinase after vigorous exercise in professional athletes. Am J Cardiol. 1984;53:856–58. [PubMed: 6702638]
- *Johnston CC, Bolton EC. Cardiac enzymes. Ann Emerg Med. 1982;11:27–35. [PubMed: 7034597]
- Karmen A, Wroblewski F, La Due JS. Transaminase activity in human blood. J Clin Invest. 1955;34:126–31. [PubMed: 13221663]
- Lee TH, Goldman L. Serum enzyme assays in the diagnosis of acute myocardial infarction. Recommendations based on a quantitative analysis. Ann Int Med. 1986;105:221–33. [PubMed: 3524337]
- *Lott JA, Stang JM. Serum enzymes and isoenzymes in the diagnosis and differential diagnosis of myocardial ischemia and necrosis. Clin Chem. 1980;26:1241–50. [PubMed: 6994925]
- Morelli RL, Carlson JC, Emilson B. et al. Serum creatine kinase MM isoenzyme sub-bands after acute myocardial infarction in man. Circulation. 1983;67:1283–89. [PubMed: 6851022]
- Roberts R. The two out of three criteria for the diagnosis of infarction. Is it passe? Chest. 1984;86:511–13. [PubMed: 6478887]
- Roberts R, Henry PD, Sobel BE. An improved basis for enzymatic estimation of infarct size. Circulation. 1975;52:743–54. [PubMed: 1236776]
- Rothkopf M, Boerner J, Stone MJ. et al. Detection of myocardial infarction extension by CK–B radioimmunoassay. Circulation. 1979;59:268–74. [PubMed: 758995]
- Vasudevan G, Mercer DW, Varat MA. Lactic dehydrogenase isoenzyme determination in the diagnosis of acute myocardial infarction. Circulation. 1978;57:1055–57. [PubMed: 639224]
Copyright © 1990, Butterworth Publishers, a division of Reed Publishing.
You have a lot of confidence and suggestions in your post.So nice.
Trả lờiXóaMark Holland
Magnesium Assay Kit