NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Bookshelf ID: NBK203 PMID: 21250047
Alkaline Phosphatase
Definition
Alkaline phosphatase (ALP) refers to a group of phosphomonoesterases that hydrolyze phosphate esters with optimum in vitro activity at a pH of 10. Enzyme activity is expressed in international units (IU), the amount of enzyme that catalyzes the conversion of 1 μmole of substrate per minute. Reference ranges vary widely with methodology; the most commonly used method produces a reference range of 35 to 125 IU per liter in an adult population. Intraindividual variation in ALP activity has been shown to vary considerably less than the interindividual variation included in reference ranges. Therefore comparison with preestablished values by the same method in an individual may be the most useful reference.Technique
Numerous methods utilizing different substrates and reaction conditions have been applied to measurement of ALP activity. The heterogeneity of methods presents problems with comparing results from different laboratories and often compromises the current application of data previously reported in the literature.Recent methodologic changes have emphasized the need for standardization and optimization of the assay. Readily hydrolyzed, self-indicating substrates that permit continuous monitoring under optimized reaction conditions are now preferred. Earlier methods (Bodansky, King, and Armstrong), which involved indirect quantitation of reaction products, are obsolete.
The most widely used reference method utilizes 4-nitro-phenyl phosphate, a readily hydrolyzed, self-indicating ALP substrate introduced in 1946 by Bessey, Lowry, and Brock. The reaction rate is enhanced by removal of inhibitory phosphate and optimizing divalent cation (magnesium and zinc) concentrations.
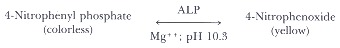
Basic Science
Most human tissues contain ALP; kidney, liver, bone, intestine, reticuloendothelial tissue, and placenta are particularly rich sources. Enzymes derived from different tissues share catalytic properties, but differ in their chemical and physical characteristics. Three genetically determined forms have been described: intestinal, placental, and liver/bone/kidney/reticuloendothelial. Different molecular properties within these isoenzyme groups are probably the result of posttranslational modifications. Various methods have been used to separate organ-specific isoforms. None are entirely satisfactory, particularly in quantifying components of the liver/bone isoenzyme group.ALP activity in normal adult serum is derived predominantly from hepatic, osseous, and reticuloendothelial sources. Physiologic bone growth in children increases serum activity to 2 to 5 times the levels observed in adults. Placental ALP causes 2-to 3-fold elevations during the second and third trimesters of normal pregnancy. Serum enrichment by intestinal isoenzyme may cause postprandial elevation, particularly in individuals with blood groups B or O who are also secretors of ABH blood group substance.
A number of functions have been proposed. The location of hepatic ALP on the sinusoid membrane suggests involvement in transport function. Increased ALP synthesis is observed during cholestasis with increased bile acid concentration caused by intrahepatic or extrahepatic obstruction of the biliary tree. Bone ALP is probably involved with calcification, perhaps by hydrolyzing pyrophosphate, which inhibits mineralization. In hypophosphatasia, an inherited disorder with virtual absence of the bone ALP isoenzyme, there is histochemical evidence for deficient bone calcification. The intestinal isoenzyme appears to function in the transport of fatty acids, calcium, and phosphate.
Clinical Significance
The majority of sustained elevated ALP levels are associated with disorders of the liver or bone, or both. Therefore, these organ systems are of prime consideration in the differential diagnosis.A variety of primary and secondary hepatic conditions may be associated with elevated serum ALP levels. Since production is increased in response to cholestasis, serum ALP activity provides a sensitive indicator of obstructive and space-occupying lesions of the liver. The latter includes neoplastic (primary or metastic) and infiltrative diseases (granulomatous hepatitis). Bilirubin excretion is compromised only with extensive biliary obstruction or diffuse hepatic cell disruption; therefore, differential elevation of ALP relative to serum bilirubin provides an early indicator for obstructive or space-occupying conditions. Hepatic cell lesions are manifested by hyperbilirubinemia and dominant serum elevation of parenchymal enzymes, such as aminotransferases; ALP elevations may be only minimal.
Diseases of bone associated with increased serum ALP are restricted to the presence of osteoblastic activity. Elevations are generally detectable prior to roentgenographic abnormalities. Neoplasms involving bone may be associated with marked serum elevations when lesions incite osteoblastic reaction, such as metastatic adenocarcinoma of the prostate. Conversely, osteolytic lesions such as occur within multiple myeloma are not associated with increased serum ALP activity. Metabolic bone diseases usually associated with serum enrichment by the bone isoenzyme include rickets, osteomalacia, and Paget's disease. Levels are usually normal in osteoporosis. Increased serum activity may be observed after bone fractures, rising after 1 week and persisting for up to 3 months.
Elevated serum ALP occurring with neoplastic disease may be due to hepatic metastases, bone metastases, or direct contribution by neoplastic cells. Isoenzymes with physicochemical characteristics similar to the placental isoenzyme have been attributed to ectopic production by a variety of neoplasms; this apparently represents derepression of normally repressed fetoplacental genes.
Clinically obscure elevations of ALP are commonly observed when multitest biochemical panels are performed on hospital populations. Because of the cellular distribution of ALP, increased serum activity may be caused by a wide variety of disorders involving multiple organs. Attempts to define organ source by isoenzyme study may be met with limited success because of technical limitations; accurate measurement of different isoenzymes contributing to total serum ALP activity is not currently possible. However, the presence of the intestinal or placental isoenzyme may be revealed by selected methods. Evaluation of an unexpectedly increased ALP should include the following:
- Exclude physiologic causes. Is the patient pregnant or an actively growing child?
- Observe for the presence of clinical or biochemical clues to the origin of increased enzyme activity. Increased serum bilirubin or aminotransferase (either aspartate or alanine) activity suggests hepatic rather than bone origin. Disproportionate elevation of lactic dehydrogenase (LDH) relative to transaminase usually suggests nonhepatobiliary or multiorgan system disease. The association of elevated LDH, hypercalcemia, and hyperuricemia suggests metastastic neoplastic disease.
- Is the elevation transient such as observed in various tissue reparative processes, healing bone fractures, or passive congestion of the liver?
- Measurement of other enzymes such as 5′-nucleotidase or gamma-glutamyltransferase may assist with identifying the hepatobiliary system as a source of elevated ALP since these enzymes are not significantly present in bone. 5′-Nucleotidase is a highly specific but less sensitive indicator of hepatobiliary disease. Gamma glutamyltransferase is more sensitive; however, with the exception of its absence in bone and placenta, it is a less specific indicator of hepatobiliary disease than ALP.
Gamma Glutamyltransferase
Definition
Gamma glutamyltransferase (GGT) is one of a broad group of enzymes that catalyze the transfer of amino acids from one peptide to another amino acid or peptide. This enzyme is sometimes referred to as a "transpeptidase" but is more appropriately included in the amino acid transferase group. Specifically it catalyzes the transfer of a gamma glutamyl group to another acceptor. The reference range with most commonly used methods is 0 to 50 IU/L in males and 0 to 30 IU/L in females. Higher activity in males is probably caused by high enzyme concentration in prostatic tissue.Technique
Self-indicating substrates with continuous reaction monitoring are preferred. Most commonly gamma-glutamyl-para-nitroanilide is used as substrate and glyclyglycine as acceptor. The substrate residue, para-nitroaniline, is directly measured by absorbance at 405 nm. The recently proposed reference method utilizes a carboxyl derivative of gamma-glutamyl-para-nitroanilide to enhance substrate solubility; higher levels of activity are observed with this substrate derivative. However, most laboratories use the unsubstituted substrate because of established clinical and laboratory experience.Serum is the specimen of choice; hemoglobin may interfere spectrophotometrically and hemolysis should be avoided. EDTA plasma is acceptable; other anticoagulants such as heparin, citrate, or oxalate may interfere with the reaction.
Basic Science
High concentrations of GGT are found in renal, prostatic, pancreatic, and hepatobiliary tissue; smaller amounts are found in all other tissues except muscle. A number of physiologic functions have been suggested. Involvement in amino acid transport and glutathione metabolism are most strongly indicated. Enzyme activity observed in serum is electrophoretically heterogeneous; however, true isoenzyme forms have not been defined, and fractionation has no clinical significance.Clinical Significance
Hepatobiliary disease is the predominant source of increased serum GGT activity. Increases are associated with all forms of primary and secondary hepatobiliary disorders. Elevations are moderate (2 to 5 times reference) with diffuse hepatic cell injury due to toxic or infectious hepatitis. Cholestasis due to intrahepatic or extrahepatic biliary obstruction causes higher serum levels (5 to 30 times reference). Increases occur earlier and persist longer than ALP in cholestatic disorders. Since skeletal disease is not associated with increased serum activity, measurement of GGT is of clinical value in identifying the source of obscure ALP elevations. Levels in children after age 1 year and healthy pregnant women are within the usual adult reference range.Elevated serum levels of GGT are also found in alcoholics and patients receiving certain drugs, such as phenytoin or phenobarbital. This is probably the result of microsomal induction of enzyme activity. Serum measurement can be used to monitor alcoholic patients during therapy; abstinence from alcohol is associated with decrease in serum GGT activity. In addition, alcohol-induced hepatic cell injury may cause significantly higher serum levels than other causes of parenchymal disorders.
Elevated GGT activity also occurs in patients with acute and chronic pancreatitis. Prostatic adenocarcinoma may be associated with increased serum levels. Therefore, although increases are absent with skeletal diseases, GGT activity should not be considered a highly specific indicator of hepatobiliary disease.
References
- Kaplan MM. Alkaline phosphatase. Gastroenterology. 1972;62:452–68. [PubMed: 4551808]
- Rosalki SB. Gamma-glutamyl transpeptidase. Adv Clin Chem. 1975;17:53–107. [PubMed: 236637]
- Wolf PL. Clinical significance of an increased or decreased serum alkaline phosphatase level. Arch Pathol Lab Med. 1978;102:497–501. [PubMed: 30431]
Copyright © 1990, Butterworth Publishers, a division of Reed Publishing.
γ-glutamyl transferase is an enzyme that transfers gamma-glutamyl functional groups. It is found in many tissues, the most notable one being the liver, and has significance in medicine as a diagnostic marker. γ glutamyltransferase
Trả lờiXóa